Saturday, January 30, 2010
Types of Heart Disease
Coronary heart disease refers to the failure of the coronary circulation to supply adequate circulation to cardiac muscle and surrounding tissue. Coronary heart disease is most commonly equated with Coronary artery disease although coronary heart disease can be due to other causes, such as coronary vasospasm.
Coronary artery disease is a disease of the artery caused by the accumulation of atheromatous plaques within the walls of the arteries that supply the myocardium. Angina pectoris (chest pain) and myocardial infarction (heart attack) are symptoms of and conditions caused by coronary heart disease.
Over 459,000 Americans die of coronary heart disease every year. In the United Kingdom, 101,000 deaths annually are due to coronary heart disease.
Cardiomyopathy
Main article: Cardiomyopathy
Cardiomyopathy literally means "heart muscle disease" (Myo= muscle, pathy= disease) It is the deterioration of the function of the myocardium (i.e., the actual heart muscle) for any reason. People with cardiomyopathy are often at risk of arrhythmia and/or sudden cardiac death.
* Extrinsic cardiomyopathies – cardiomyopathies where the primary pathology is outside the myocardium itself. Most cardiomyopathies are extrinsic, because by far the most common cause of a cardiomyopathy is ischemia. The World Health Organization calls these specific cardiomyopathies[citation needed]:
o Alcoholic cardiomyopathy
o Coronary artery disease
o Congenital heart disease
o Nutritional diseases affecting the heart
o Ischemic (or ischaemic) cardiomyopathy
o Hypertensive cardiomyopathy
o Valvular cardiomyopathy – see also Valvular heart disease below
o Inflammatory cardiomyopathy – see also Inflammatory heart disease below
o Cardiomyopathy secondary to a systemic metabolic disease
* Intrinsic cardiomyopathies – weakness in the muscle of the heart that is not due to an identifiable external cause.
o Dilated cardiomyopathy (DCM) – most common form, and one of the leading indications for heart transplantation. In DCM the heart (especially the left ventricle) is enlarged and the pumping function is diminished.
o Hypertrophic cardiomyopathy (HCM or HOCM) – genetic disorder caused by various mutations in genes encoding sarcomeric proteins. In HCM the heart muscle is thickened, which can obstruct blood flow and prevent the heart from functioning properly.
o Arrhythmogenic right ventricular cardiomyopathy (ARVC) – arises from an electrical disturbance of the heart in which heart muscle is replaced by fibrous scar tissue. The right ventricle is generally most affected.
o Restrictive cardiomyopathy (RCM) – least common cardiomyopathy. The walls of the ventricles are stiff, but may not be thickened, and resist the normal filling of the heart with blood.
o Noncompaction Cardiomyopathy – the left ventricle wall has failed to properly grow from birth and such has a spongy appearance when viewed during an echocardiogram.
Cardiovascular disease
Main article: Cardiovascular disease
Cardiovascular disease is any of a number of specific diseases that affect the heart itself and/or the blood vessel system, especially the veins and arteries leading to and from the heart. Research on disease dimorphism suggests that women who suffer with cardiovascular disease usually suffer from forms that affect the blood vessels while men usually suffer from forms that affect the heart muscle itself. Known or associated causes of cardiovascular disease include diabetes mellitus, hypertension, hyperhomocysteinemia and hypercholesterolemia.
Types of cardiovascular disease include:
* Atherosclerosis
Ischaemic heart disease
* Ischaemic heart disease – another disease of the heart itself, characterized by reduced blood supply to the organs.
Heart failure
Main article: Heart failure
Heart failure, also called congestive heart failure (or CHF), and congestive cardiac failure (CCF), is a condition that can result from any structural or functional cardiac disorder that impairs the ability of the heart to fill with or pump a sufficient amount of blood throughout the body. Therefore leading to the heart and body's failure.
* Cor pulmonale, a failure of the right side of the heart.
Hypertensive heart disease
Main article: Hypertensive heart disease
Hypertensive heart disease is heart disease caused by high blood pressure, especially localised high blood pressure. Conditions that can be caused by hypertensive heart disease include:
* Left ventricular hypertrophy
* Coronary heart disease
* (Congestive) heart failure
* Hypertensive cardiomyopathy
* Cardiac arrhythmias
Inflammatory heart disease
Inflammatory heart disease involves inflammation of the heart muscle and/or the tissue surrounding it.
* Endocarditis – inflammation of the inner layer of the heart, the endocardium. The most common structures involved are the heart valves.
* Inflammatory cardiomegaly
* Myocarditis – inflammation of the myocardium, the muscular part of the heart.
Valvular heart disease
Main article: Valvular heart disease
Valvular heart disease is disease process that affects one or more valves of the heart. There are four major heart valve which may be affected by valvular heart disease, including the tricuspid and aortic valves in the right side of the heart, as well as the mitral and aortic valves in the left side of the heart.
Heart Disease and Cardiac Catheterization
Why Do I Need a Cardiac Cath?
Your doctor uses cardiac cath to:
* Evaluate or confirm the presence of heart disease (such ascoronary artery disease, heart valve disease, or disease of the aorta).
* Evaluate heart muscle function.
* Determine the need for further treatment (such as an interventional procedure or bypass surgery)
At many medical centers, several interventional, or therapeutic, procedures to open blocked arteries are performed after the diagnostic part of the cardiac cath is complete. Interventional procedures include balloon angioplasty and stent placement. Rarely, more complicated procedures, such as brachytherapy, atherectomy, rotoblation, and cutting balloon are done.
What Are the Risks Associated With Cardiac Cath?
A cardiac cath is generally safe. However, as with any invasive procedure, there are risks. Special precautions are taken to decrease these risks. Your doctor will discuss the risks of the procedure with you.
Risks of a cardiac cath are rare but can include:
* Bleeding around the point of puncture
* Abnormal heart rhythms
* Blood clots
* Infection
* Allergic reaction to the dye
* Stroke
* Heart attack
* Perforation of a blood vessel
* Air embolism (introduction of air into a blood vessel, which can be life-threatening)
* Death
Be sure to ask your doctor any questions you may have before undergoing the procedure.
How Should I Prepare for a Cardiac Cath?
For a cardiac cath, most people will need to have a routine chest X-ray, blood tests, electrocardiogram, and urinalysis performed within two weeks beforehand.
You can wear whatever you like to the hospital. You will wear a hospital gown during the procedure.
Leave all valuables at home. If you normally wear dentures, glasses, or a hearing device, plan to wear them during the cardiac cath.
Your doctor or nurse will give you specific instructions about what you can and cannot eat or drink before the procedure.
Tell your doctor all of the medications you are currently taking, including herbal products and dietary supplements.
Ask your doctor what drugs should be taken on the day of your cardiac cath. You may be told to stop taking certain medications, such as Coumadin (a blood thinner), for a few days before the procedure.
If you have diabetes, ask your doctor how to adjust your diabetes drugs the day of your test.
Tell your doctor and/or nurses if you are allergic to anything, especially iodine, shellfish, X-ray dye, latex, or rubber products (such as rubber gloves or balloons) or penicillin-type medications.
You may or may not return home the day of your procedure. Bring items with you (such as a robe, slippers, and toothbrush) to make your stay more comfortable. When you are able to return home, arrange for someone to bring you home.
How Long Does a Cardiac Cath Last?
A cardiac cath procedure usually takes about 30 minutes, but the preparation and recovery time add several hours. Plan on being at the hospital all day for the procedure.
What Happens During a Cardiac Cath?
You will be given a hospital gown to wear for a cardiac cath. A nurse will start an intravenous (IV) line in your arm so that medications and fluids can be administered through your vein during the procedure.
The cardiac cath room is cool and dimly lit. You will lie on a special table. If you look above, you will see a large camera and several TV monitors. You can watch the pictures of your cardiac cath on the monitors.
The nurse will clean your skin (and possibly shave) the site where the catheter will be inserted (arm or groin). Sterile drapes are used to cover the site and help prevent infection. It is important that you keep your arms and hands down at your sides and not disturb the drapes.
Electrodes (small, flat, sticky patches) will be placed on your chest. The electrodes are attached to an electrocardiogram (ECG) machine that charts your heart's electrical activity.
A urinary catheter may be necessary for the procedure.
You will be given a mild sedative to help you relax, but you will be awake and conscious during the entire procedure. The doctor will use a local anesthetic to numb the catheter insertion site.
If the catheter is to be inserted at the groin (called the "femoral" approach), a local anesthetic will be injected to numb the area. A small incision will be made over the blood vessel through which the catheter and introducer sheath will be inserted. The catheter will be inserted through the sheath and threaded to the arteries of your heart. Again, if you feel pain, tell your health care providers.
If the catheter is to be inserted into your arm (at the bend of the elbow, called the "brachial" approach), a local anesthetic will be injected into the skin in your arm to numb the area. A small incision will be made over the blood vessel through which the catheter introducer sheath (a tube through which the catheter is passed) and catheter will be inserted. The catheter will be inserted through the sheath and threaded to the arteries of your heart. Although you may feel pressure as the incision is made or when the sheath and catheter are inserted, you should not feel pain; tell your health care providers if you do.
When the catheter is in place, the lights will be dimmed and a small amount of dye (or "contrast material") will be injected through the catheters into your arteries and heart chambers. The contrast material outlines the vessels, valves, and chambers.
What Happens During a Cardiac Cath? continued...
When the contrast material is injected into your heart, you may feel hot or flushed for several seconds. This is normal and will go away in a few seconds. Please tell the doctor or nurses if you feel itching or tightness in the throat, nausea, chest discomfort, or any other symptoms.
The X-ray camera will be used to take photographs of the arteries and heart chambers. Your doctor may ask you to take a deep breath, hold your breath, or to cough during the procedure You will be asked to hold your breath while the X-rays are taken. When all the photos have been taken, the catheter will be removed and the lights will be turned on.
What Happens After the Cardiac Cath?
If the catheter was inserted in your groin, the introducer sheath will be removed and the incision will be closed with stitches, a collagen seal, or applied pressure. In some situations, the introducer sheath may be stitched into place and removed after the bleeding stops. A collagen seal is a protein material that works with your body's natural healing processes to form a clot in the artery.
If the catheter was inserted in your arm, the catheter and sheath are removed. The incision will be closed with stitches and bandaged. You will need to keep your arm straight for at least an hour. You will be able to walk around. You will be observed for a few hours to make sure you are feeling well after the procedure. You may receive medication to relieve discomfort in your arm after the anesthetic wears off. You will be given instructions regarding how to care for your arm when you return home. Tell your nurse if you think you are bleeding or feel any numbness or tingling in your fingers.
A sterile dressing will be placed on the groin area to prevent infection. You will need to lay flat and keep the leg straight for two to six hours to prevent bleeding. Your head can not be raised more than two pillows high (about 30 degrees). Do not raise your head off the pillows, as this can cause strain in your abdomen and groin. Do not try to sit or stand. The nurse will check your bandage regularly, but tell your nurse if you think you are bleeding (have a wet, warm sensation) or if your toes begin to tingle or feel numb. You may receive medication to relieve discomfort in the groin area after the anesthetic wears off. Your nurse will help you out of bed when you are allowed to get up.
Your doctor's orders will determine when you will be allowed out of bed to go to the bathroom. You will need assistance getting out of bed, so ask for help. The nurse will help you sit up and dangle your legs on the side of the bed.
What Happens After the Cardiac Cath? continued...
You will need to drink plenty of liquids to clear the contrast material from your body.
You may feel the need to urinate more frequently. This is normal. If a urinary catheter was not placed during the procedure, you will need to use a bedpan or urinal until you are able to get out of bed.
Your doctor will tell you if you are able to return home or will need to stay overnight. In either case, you will be monitored for several hours after the procedure.
Treatment, including medications, dietary changes, and future procedures will be discussed with you prior to going home. Care of the wound site, activity, and follow-up care will also be discussed.
Please ask your doctor if you have any questions about cardiac cath.
Friday, January 29, 2010
Atresia and stenosis of the colon (2)
Treatment
Medical Therapy
Initial treatment of newborns with colonic atresia is directed toward resuscitation. The child is often distended and dehydrated. Usual treatments prior to operative intervention include the following:
- Nasogastric decompression
- Intravenous fluid resuscitation
- Intravenous antibiotics
Surgical Therapy
The management of colonic atresia is directed at eliminating the bowel obstruction and establishing intestinal continuity. In selected cases (with limited comorbidities and limited associated malformations), this may be performed in one operation by resecting the atretic ends and anastomosing the colon. If the child has significant comorbidities, a diverting enterostomy may be brought out just proximal to the atresia, and intestinal continuity may be restored during a second operation.
Many authors advocate resection with primary anastomosis for right colon lesions and colostomy diversion with subsequent reconstruction for left-sided atresia and stenosis. Theoretically, the liquid feces in the right colon pose less risk to a fresh anastomosis than formed stool. However, in practice, Davenport et al noted that the type of procedure had no effect on survival rates, and outcomes were excellent using either technique.5
Colonic atresia associated with Hirschsprung disease is often, but not exclusively, associated with abnormal distal colonic fixation.43,29 The association of nonfixation with aganglionosis seems significant enough to warrant obtaining biopsy results prior to establishing intestinal continuity in those selected cases. Diverting enterostomy alone is always a safe option if the suspicion of aganglionosis is significant and experienced pediatric pathology services are unavailable.
In congenital colonic stenosis, one usually finds less difference in the sizes of the proximal and distal limbs, making resection with primary anastomosis the preferred treatment.
In acquired colonic stenosis following necrotizing enterocolitis, primary excision and anastomosis is the treatment of choice for stable patients without life-threatening comorbidities.
Preoperative Details
Basic preoperative laboratory measurements for all neonates should include a CBC count, electrolyte assessment, and a crossmatch. If time permits, a contrast enema can be quite useful in assessing the colon and excluding any other narrowings in that area, which can sometimes be difficult intraoperatively.
A rectal biopsy should be performed if possible to eliminate the slight chance of Hirschsprung disease, but all care should be taken to ensure that it does not delay prompt operative intervention.
Intraoperative Details
Primary repair in one stage
- Laparotomy: A transverse supraumbilical laparotomy is usually performed. The abdomen is eviscerated, and the bowel is inspected. The atresia should be readily visible by the dilated bulbous proximal portion and a microcolon on the other side. A large mesenteric or intestinal gap may be observed between the proximal and distal ends, reflective of the region that would have been supplied by the vessel that had the prenatal vascular accident, which hypothetically caused the atresia.
- Full exploration: The abdomen must now be fully explored. The full intestinal length must be examined for the presence of other atresias. This may require passing a catheter distally into the open end of the distal segment and distending the bowel with saline to ensure the lumen is patent. The presence of multiple atresias may require multiple resections and should be assessed together to minimize intestinal loss and the number of anastomoses.
- Proximal segment: In preparing to put the two ends of the colon together, the dilated proximal portion must be resected. The dilated proximal bowel functions poorly due to dysmotility. Resecting the proximal intestine back to an area of normal caliber is essential for postoperative function and lessens the size discrepancy when performing the anastomosis.
- Distal segment: Minimal distal bowel is removed, although the atretic end is thickened and should not be used in closure. That end is resected, and the distal colon is usually divided on a bias, removing more bowel on the antimesenteric side than the mesenteric side (to promote better blood supply at the anastomosis). The antimesenteric side is usually opened further to counter the size discrepancy with the proximal end.
- Mesenteric gap: If present, the mesenteric gap is usually closed primarily, if possible.
Diversion with primary repair at a second stage
The operation is much the same as for primary repair above.
- Proximal segment: The proximal end of the atresia is again resected back to a normal-caliber bowel. This end is then brought out onto the anterior abdominal wall and matured as an ostomy.
- Distal segment: If the patient is critically ill, the distal segment may be left completely undisturbed until the patient's status changes in the future. For stable patients, the distal end of the atresia should have its end removed, and the fresh end should be brought up to the abdominal wall skin as a mucous fistula.
- Ostomy location and maturation: Both ostomies can be brought out at the same location, which may be at the corner of the laparotomy incision or may be through a separate incision. They may also be separated by a skin bridge; however, that is nonessential. What is most important is that the colostomy be matured and positioned on the abdominal wall in such a way that an appliance can be securely placed around it.
- Mucous fistula: The mucous fistula should not be matured like a mushroom but should be flush with the skin and should only be large enough to be able to admit a catheter for irrigations or contrast radiography. If it is placed immediately next to the colostomy with no skin bridge, it should be small enough to allow appliance placement over both. If the surgeon prefers a skin bridge between the ostomy and mucous fistula, it should be either small enough to be directly covered by the ostomy appliance wafer or should be far enough away as to not interfere with ostomy appliance placement.
Postoperative Details
The neonate who has undergone surgical correction of atresia or stenosis requires neonatal critical care in conjunction with the pediatric surgical care.
In the early postoperative period, the intestine and soft tissues absorb fluid, which surgeons refer to as third-space losses. This requires significant hydration to ensure the maintenance of intravascular volume and adequate tissue perfusion. Inadequate hydration can lead to pressor administration, which can impair intestinal perfusion. Third-space losses usually persist for 24-48 hours if no source of sepsis is present.
All children who undergo laparotomy need adequate analgesia. Narcotics should only be administered to babies in a monitored setting (ie, a neonatal intensive care unit with apnea, pulse-oximetry, and cardiac monitors). No baby should be prevented from getting adequate analgesia simply because they cannot articulate the need for it.
Antibiotics are usually administered to all patients, although the indications are not clearly evidence based. Within the first 3 days of life, the intestinal flora is not yet colonized with anaerobes, so gram-positive and gram-negative coverage is sufficient at that time.
Early parenteral nutrition should be started in all patients. Patients with atresia, despite having their bulbous dysmotile proximal portions resected, can have prolonged postoperative ileus and usually benefit from early parenteral nutrition.
If a nasogastric tube was placed during the operation, it usually remains in place at the discretion of the surgeon. The most traditional approach is to keep a nasogastric tube to suction (taking care to replace its output with intravenous fluids) until the child is passing flatus or stool. When that occurs, the tube may be placed to bedside gravity drainage; if that is well tolerated, the tube may be removed. Some surgeons clamp the tube prior to removal as a final test to see if the child can truly tolerate their own intestinal gases and secretions.
When the tube is removed, the diet may be started. Most surgeons start with clear liquids, such as sugar water or a balanced electrolyte solution. When that is well tolerated, the diet is advanced to either breast milk or formula.
Patients who have undergone a colostomy require specific care for the stoma and its output. Return of bowel function in these patients is usually faster after colostomy than in those who undergo resection and anastomosis. Colostomy closure is performed electively at a future date. Most surgeons prefer to wait a minimum of 6-8 weeks to allow intraperitoneal inflammation to subside.
Follow-up
Follow-up imaging studies are not performed unless indicated by clinical issues. Children who have had a stoma created usually undergo contrast enema prior to stomal closure to evaluate the distal limb for stricture or obstruction.
Follow-up in the office is essential. Many of these babies have residual intestinal motility problems that may manifest in either the proximal or distal segments. Close follow-up is essential to ensure that they are appropriately managed.
Complications
Complications of surgery for colonic atresia and stenosis are those of any bowel resection with stoma creation or anastomosis. Wound infection or incisional hernia can occur after any surgery.
The complication surgeons are most concerned about is anastomotic leak. Leaks may manifest at any time but usually become noticeable several days to a week after surgery. Signs may be as subtle as mild tachycardia and fever, or the patient may rapidly progress from mild illness into full-blown septic shock. Radiography may reveal intra-abdominal free air. CT scanning could reveal abscesses. Anastomotic leak requires exploration and diverting enterostomy.
Intra-abdominal abscesses may occur without leak but are rare. Intraoperative bleeding that leads to postoperative clot can be a setup for abscess, which may be amenable to drainage by interventional radiology.
Anastomotic narrowing may occur and may be related to technical error, ischemia, or leakage.
Patients who undergo colostomy may have stoma narrowing, prolapse, or parastomal herniation.
Wednesday, January 27, 2010
Atresia and stenosis of the colon (1)
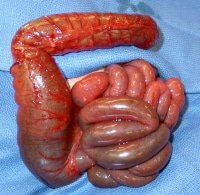
The term colonic atresia describes a condition in which a part of the colon has failed to correctly form, and that part is either completely blocked or is altogether missing. Colonic stenosis describes a condition in which a part of the colon is very narrow, resulting in a partial blockage. Other obstructions of the colon that affect newborns include Hirschsprung disease and small left colon syndrome, as well as obturation obstruction, meconium ileus, or meconium plug. These issues are all forms of colonic obstruction; however, they are different from atresia and stenosis and are more completely reviewed in their own articles.
The colon is the rarest site of atresia in the GI tract. It is a congenital anomaly which may be suggested using prenatal ultrasonography and is usually revealed in affected newborns shortly after birth. Patients usually present with abdominal distention and failure to pass meconium.
Stenosis of the colon is much more common. Patients usually present later in life, most often because of an identifiable event. In congenital stenosis, a narrow segment of colon is observed, but bowel continuity is maintained. A discrepancy between the colonic segments above and below the area of stenosis is present. In acquired stenosis, which is commonly referred to as stricture, what starts as a normal segment becomes narrowed. This is most common in premature babies who have recovered from an episode of necrotizing enterocolitis.
History of the Procedure
Colonic atresia was first recorded in 1673.1 The first survivor was reported by Gaub in 1922 and was treated using a diverting colostomy.2 Potts reported the first survival following primary repair in 1947.3
Problem
The common problem in both atresia and stenosis is intestinal blockage, either partial or complete.
In colonic atresia, the problem is complete bowel obstruction. Gas and stool cannot pass, and the colonic segment above the atresia becomes distended. If left untreated, this leads to perforation.
In colonic stenosis, the problem is that gas and stool try to pass through a narrow area. While the baby is passing soft baby stools, this may or may not be noticeable. When the baby's diet changes from breast milk or formula to cereals and solid foods, the stool can become thicker and more formed. This may cause stenosis to become symptomatic, leading to distension, feeding intolerance, or failure to thrive.
In babies who have had necrotizing enterocolitis, stenosis occurs after the original episode has resolved. This may manifest in varying degrees, ranging from minor feeding intolerance and distension to near-complete bowel obstruction.
Frequency
Colon atresia is very rare. Various incidences have been reported, ranging from 1 in 1500 live births4 to 1 in 66,000 live births.5 Webb (1931) and Benson (1968) cited the incidence as 1 in 20,000, which is most reflective of experiences in the modern era.6,7 In a 1982 report, Powell suggested that colonic atresia represents 5-15% of intestinal atresias,8 whereas in 1966, Freeman reported a lower figure of 1.8%.9 In 1953, Gross recorded 6 cases of colonic atresia out of 140 cases of intestinal atresia (4.3%) at the Boston Children's Hospital.10
Multiple atresias are uncommon in the colon; however, colonic atresia may be overlooked when small intestinal atresia is present.11 Rare cases of familial colonic atresia have been described. Animal studies have shown an autosomal recessive pattern of inheritance in cattle. Hereditary multiple intestinal atresia affects both the large and small intestine, whereas nonhereditary multiple intestinal atresia usually spares the colon.12
The incidence of colonic stenosis is not readily available because most cases are acquired. In 1953, Gross noted a single colonic lesion in 71 patients with intestinal stenosis.10 Necrotizing enterocolitis is the most common etiology of postnatal colonic stenosis; narrowing develops in 10-25% of affected patients.13,14,15
Etiology
See Relevant Anatomy.
Pathophysiology
See Relevant Anatomy.
Presentation
Patients with colonic atresia or congenital stenosis may sometimes have findings on prenatal ultrasonography, such as dilated bowel loops or the presence of polyhydramnios. Initial physical examination findings are normal in the absence of associated conditions. The anus usually appears normal. Progressive abdominal distention develops. Rectal examination reveals white or pale mucus rather than pigmented meconium. Failure to completely pass meconium suggests atresia, whereas delayed passage of meconium (>24 h) suggests Hirschsprung disease. Patients with colonic atresia may pass meconium normally because the incident that caused the atresia may have occurred after the colon had become filled with meconium.
Colonic stenosis usually follows some form of injury to the colon, and ischemia is considered central to the insult. This injury may be in utero or postnatal. The infant or child may present with symptoms similar to atresia with high-grade stenosis; less stenotic lesions may not become apparent until feeding is undertaken. In those instances, the child's abdomen may become distended with feeding, and stool production is scant, if present.
Babies with necrotizing enterocolitis may show signs of acquired stenosis following their acute episode. When the septic signs of the illness resolve and the child is doing well, feeding is often attempted. Those babies who have formed stenoses usually do not tolerate feeds and become distended. Studies may be performed to confirm the diagnosis and to try to localize the site of narrowing, at the discretion of the surgeon.
Associated conditions
Colonic atresia has been associated with abdominal wall defects and abnormalities of the genitourinary tract.16 Nonfixation of the colon has been reported.17 Association with anal atresia18 and imperforate anus19 has been reported but is extremely rare. Colonic perforation may occur.20 This is thought to be caused by overdistension of closed colonic loop, with gas and stool trapped between a competent ileocecal valve proximally and the blind-ending colon distally. In 1988, Pohlson et al reported perforation of the terminal ileum in one case, clearly demonstrating that perforation can occur anywhere proximal to the obstructed bowel.21
Hirschsprung disease has been present in a small number of cases.22,23,24,25 Additional anomalies associated with this pairing include omphalocele26 and absence of a hand.27 In most cases the aganglionosis is discovered after colostomy closure when the distal bowel does not properly function. Some authors have recommended that rectal biopsy be performed at the time of laparotomy,28 whereas others believe that biopsy should be reserved for children who do not pass stool readily upon restoring bowel continuity after resection.23,25 Because colonic atresia is rare and because of the morbidity and mortality associated with missing the diagnosis prior to establishing intestinal continuity, rectal biopsy prior to definitive repair would seem prudent.29
Cardiac conditions that require catheterization may predispose a baby to a mesenteric vascular incident resulting in colonic stenosis. Additional conditions reported in patients with colonic stenosis include cryptophthalmia syndrome (ie, cleft lip and palate, microphthalmia, dysplastic kidneys, proximal jejunal atresia), arthrogryposis, proximal intestinal atresia, neoplasm, and malrotation.4,8 Riley-Day syndrome (ie, familial dysautonomia) has been associated with spontaneous colon ischemia.30 Coloboma, cataracts and facial hemihypertrophy, facial asymmetry with palsy, microphthalmia with partial iridial coloboma, exophthalmia, and bilateral optic nerve hypoplasia all have been reported.8 Kim et al (2000) described a case of colonic atresia in monozygotic twins.12
Indications
Like all other intestinal atresias, colonic atresia is fatal if the obstruction is not relieved. A baby with colonic atresia is at risk for dehydration, perforation, and sepsis. Any intestinal atresia requires operative intervention to prevent these complications.
The specific operative indication in colonic atresia is complete bowel obstruction. Once a newborn is identified as having bowel obstruction, no additional information is required to prove the need for surgery. Other studies may be done prior to operation, but no studies are necessary to confirm the need for surgery.
Colonic stenosis behaves like colonic atresia when the lesion is very tight. In less severe cases, the child may have chronic problems like bloating with feeds, cramping, or poor weight gain. Any of these symptoms may be significant enough to warrant either radiological investigations or operative exploration and repair.
Relevant Anatomy
Embryology, anatomy, and pathophysiologyThe colon arises from the digestive tube, which is present by the end of the first month of gestation. Rapid elongation begins at 5 weeks' gestation. Over the ensuing 5 weeks, the intestinal tube, separable into cephalad and caudal limbs (based on the relationship to the omphalomesenteric duct), rotates counterclockwise and returns to its familiar position in the abdomen. The proximal caudal limb receives its blood supply from the superior mesenteric artery (SMA), whereas the inferior mesenteric artery (IMA) supplies the distal portion.31
The SMA gives rise to the ileocolic, right colic, and middle colic arteries, which supply the ileocecal region, the ascending colon, and the proximal transverse colon, respectively. The left colic artery, arising from the IMA, supplies the left portion of the transverse colon and the descending colon. The middle colic and left colic arteries join near the splenic flexure to form the marginal artery. The sigmoid arteries, rectosigmoid arteries, and branches of the IMA supply the sigmoid colon.
Small bowel and colonic atresias are not believed to occur by the same process as in duodenal atresia, which is suspected to be due to failure of vacuolization of the duodenum and was described by Tandler in 1900.
In 1955, Louw and Barnard hypothesized that small bowel atresias are caused by prenatal vascular interruption.32 The same mechanism is believed to cause colonic atresia. Thrombosis, volvulus, and herniation with strangulation are all mechanisms that may cause in utero vascular injury and bowel necrosis with subsequent reabsorption. Fairbanks proposed a relationship between fibroblast growth factor 10 expression, vascular development, and colonic atresia.33
Although the colon receives its blood supply from the SMA and the IMA, much variation among smaller named arterial branches is observed. The portions of the colon most vulnerable to ischemia appear to be the splenic flexure and the ileocecal area. Coincidentally, these areas are the farthest from the major trunks. Nevertheless, atresia and stenosis occur throughout the colon; the wide variation in terminal branches coupled with the complex rotation and fixation of the bowel may create regions of bowel ischemic injury in utero that result in atretic or stenotic lesions.34
Any process that leads to occlusion of branches of these vessels in utero may result in atresia. Compression at the umbilical ring,35 internal hernia, intussusception,36 choledochal cyst,37 volvulus, and thrombosis have all been implicated in the initiation of bowel infarction, leading to disintegration, gradual reabsorption of dead tissue, and sealing of the bowel ends in the fetus as described by Louw in 1964.38 The bowel content is sterile; thus, sepsis does not occur. Meconium is produced throughout the gut during the third trimester; thus, a newborn whose atresia occurs after that time may still pass meconium in the newborn period.
Prenatal maternal use of vasoconstrictive medications, such as cocaine, amphetamines, nicotine, or decongestants, has been suggested to be a risk factor for intestinal atresia formation.39
Congenital stenosis occurs when the bowel injury is incomplete. This may happen when injury occurs close to the bowel wall, allowing collateral blood flow to preserve the injured tissue. Another mechanism is limited ischemia, in which the blood supply is partially or intermittently occluded, resulting in incomplete intestinal injury.
Acquired stenosis is more common than atresia or congenital stenosis. Via the same mechanism of vascular compromise, the injured bowel undergoes healing and scarring with narrowing of the affected intestine. Intense inflammatory reactions, such as those that occur in necrotizing enterocolitis and Crohn disease, may result in stricture. Tuberculosis-associated left colon stricture has also been reported.40
Patients who undergo bowel resection for any reason and have a segment of intestine removed and joined by anastomosis may develop stricture at the anastomotic site due to ischemia or technical issue. Surgery is usually required to revise the joined loops.
Colon atresia is typically classified using the 1989 descriptions of intestinal atresia by Bland-Sutton and the 1964 descriptions by Louw.41,38 In type 1 lesions, the bowel and mesentery remain intact, but the bowel lumen is interrupted by a complete membrane (see Media file 1). Type 2 lesions are those in which the bowel is discontinuous, connected by a fibrous cord. In type 3 lesions, the bowel ends are completely separated, and the mesentery has a gap. Stenotic lesions are characterized by intact bowel with incomplete occlusion and require no classification.
In 1990, Davenport et al reviewed 118 cases of colonic atresia. The distribution of the lesions by site was as follows:5
- Ascending colon - 33 (28%)
- Hepatic flexure - 4 (3%)
- Transverse colon - 27 (23%)
- Splenic flexure - 30 (25%)
- Descending and sigmoid - 24 (20%)
Two thirds of colon atresias are in the distribution of the IMA. This may relate to lack of collateral blood supply or disease processes that render this portion of the colon more susceptible to injury.
Contraindications
Intestinal obstruction at any level requires surgical relief. The timing of surgery in colonic atresia and stenosis depends on the patient's clinical condition and their associated malformations and comorbidities.
The decision to proceed with primary correction (resection with anastomosis) or stoma diversion depends on the same factors, as well as the skill and experience of the surgeon and their team. Although no contraindications to surgery are recognized, stoma diversion is the minimum intervention necessary to relieve the obstruction. Severe underlying illness and associated life-threatening malformations may be considered relative contraindications to immediate primary repair.
Tuesday, January 26, 2010
Kolelitiasis
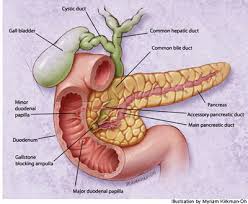 |
Causes
The main components of gallstones are cholesterol, another small portion is formed from calcium salts. Bile contains large amounts of cholesterol that usually remains as a liquid. If the bile becomes saturated because of cholesterol, so cholesterol can become insoluble and precipitate out to form bile.
Risk Factors
1. Gallstones are more common in women and risk factors are:
2. Elderly.
3. Overweight (obesity).
4. High-fat diet.
5. Heredity.
Pathophysiology
Most gallstones form in the gallbladder and the majority of stones in the bile ducts from the gallbladder. Gallstones can form in the bile duct if it has back-flow of bile due to the narrowing of the channel or after gallbladder removal.
Gallstones in the bile duct can lead to infection of the bile ducts great (cholangitis), infection of the pancreas (pancreatitis) or liver infection. If the bile duct is blocked, then the bacteria will grow and cause infection once in the channel. Bacteria can spread through the bloodstream and cause infection in other body parts.
The majority of gallstones in a long time does not cause symptoms, especially if the stones in the gallbladder settled. Sometimes large stones will gradually erode the gallbladder wall and into the small intestine or large intestine, causing intestinal obstruction (gallstone ileus).
What more often happens is out of gallstones and gallbladder into the bile ducts. Of the bile ducts, gallstones can enter the small intestine or remain in the bile ducts without causing interruption of bile flow and symptoms.
Symptoms and Signs
If gallstones suddenly clogged bile ducts, then the patient will feel pain. Pain tends to relapsing-remitting and pain known as colic. Occur slowly and reached its peak, then decreased gradually. The pain is sharp and relapsing-remitting, can last for hours. Location of pain varies, but most felt in the upper right abdomen and can spread to the right shoulder.
Patients often feel nausea and vomiting. If an infection with a blocked duct, it will have a fever, chills and jaundice (jaundice). Blockage is usually temporary and rarely infection.
Pain due to blockage of the channel can not be distinguished from pain due to gall bladder blockage. Permanent obstruction in the cystic duct causing inflammation of the gallbladder (acute cholecystitis). Gallstones that block the pancreatic duct causing inflammation of the pancreas (pancreatitis), pain, jaundice and may also be infected.
Sometimes the pain of relapsing-remitting relapsed again after gallbladder removed, pain may be caused by gallstones in the main bile duct.
Complications
Complications that may be happening is:
* Bleeding
* Inflammation of the pancreas (pancreatitis).
* Perforation or biliary tract infection.
In 2-6% of patients, shrinking the channel back and reappeared gallstones.
Prevention
Because the greatest composition of gallstones is cholesterol, should avoid foods high in cholesterol, which generally come from animal fats.
Management
If not found symptoms, then treatment is not necessary. Pain of relapsing-remitting be avoided or reduced by avoiding or reducing fatty foods.
Gallbladder stones
If the gall bladder stones cause repeated pain attacks have been carried out despite dietary changes, it is advisable to undergo gallbladder removal (cholecystectomy). Appointment of the gall bladder does not cause nutrient deficiency and after surgery is not necessary food restrictions.
Laparoscopic cholecystectomy was introduced in 1990 and is currently about 90% done in laparoscopic cholecystectomy. The gallbladder is removed through a tube inserted through a small incision in the abdominal wall. This type of surgery has the following advantages:
* Reduce discomfort after surgery.
* Shortening the period of hospitalization.
Other techniques to remove gall bladder stones are:
* Solution with methyl-butyl-ether.
* Solving the sound waves (lithotrypsy).
* Dissolving with chronic bile acid treatment (kenodiol acid and acid ursodeoksikolik).
Bile duct stones
bile duct stones can cause serious problems, because it must be removed either surgically or through an abdominal procedure called Endoscopic retrograde cholangiopancreatography (ERCP). In ERCP, an endoscope is inserted through the mouth, esophagus, stomach and into the small intestine.
Radioopak contrast substance into the bile duct through a tube in the sphincter Oddi. In sfingterotomi, sphincter muscle to open a bit wide so that gallstones are blocking the channel will move to the small intestine. ERCP and sfingterotomi has been successfully performed on 90% of cases. Less than 4 of every 1,000 patients who died and 3-7% have complications, so that this procedure is safer than abdominal surgery. ERCP is usually effective only performed on patients bile duct stones are older, who had been appointed bladder bile.
Monday, January 25, 2010
Duhamel prosedure
Although this disease was first described by Ruysch in 1691 and popularized by the Hirschsprung in 1886, patofisiologinya not known until the mid-20 th century, when Whitehouse and Kernohan get aganglionosis in the distal bowel obstruction as the cause of their patient case report. In 1949, Swenson explained the definitive management of Hirschsprung's is by rectosigmoidectomy with colonal anastomosis. After that note other types of surgery techniques, including techniques Duhamel and Soave. At present, there is progress on operating techniques, including minimal invasive procedures, and early diagnosis has reduced the mortality and morbidity of patients with Hirschsprung's disease.
Figure 1. Images of normal colon on the left and a dilated colon in Hirschsprung's disease on the right
Most cases of Hirschsprung's disease is now diagnosed in the neonatal period. Hirschsprung's disease should be suspected if a newborn does not remove meconium in the first 24-48 hours after birth. Although barium enema is useful for diagnosis, biopsy of the rectum remains the gold standard diagnosis enforcement. Once the diagnosis is confirmed, treatment is essential to remove the intestinal tissue to make aganglionik and anastomosis with the distal rectum connects with the proximal intestine that has a healthy innervasi.
Pathophysiology
Congenital Aganglionis the distal colon is understanding Hirschsprung's disease. Aganglionosis begins in the rectum, which is always affected, and continues to the proximal direction with various distances. Myenterik plexus (Auerbach) and submukosal plexus (Meissner) does not exist, causing reduction in intestinal peristaltic and other functions. Precise mechanism of the development of this disease is unknown.
Enteric ganglion cells derived from neuroblast cell differentiation. During normal development, neuroblast can be found in the small intestine at the age of 7 weeks gestation and will be up to the colon at 12 weeks gestation. One possibility is the etiology of Hirschsprung defects in neuroblast cell migration was in its path toward the distal colon. Neuorblas the normal migration can occur with the failure of the last neuroblas, berpoliferase, or berdifferensiasi on aganglionik distal segment. Distribution of components that are not proportional to neuronal growth and development has occurred in the intestine that aganglionik, these components are fibronektin, laminin, neural cell adhesion molecule, and neurotrophic factors.
In addition, observations of smooth muscle cells in the colon indicates that the aganglionik is not active when Electrophysiology tests, this shows myogenik abnormalities in the development Hirschspurng disease. Abnormal Cajal cells, which connect the pacemaker cells between enteric nerves and intestinal smooth muscle, also has dipostulat become important factors that contribute.
There are three neuronal plexuses of the intestine menginnervasi, submukosal plexus (Meissner), Intermuskuler (Auerbach), and the mucosal plexus. All three are integrated plexus and a role in all aspects of bowel function, including absorption, secretion, motility, and blood flow.
Normal motility mainly controlled by intrinsic neurons. These ganglia control the contraction and relaxation of smooth muscle, where relaxation dominates. Bowel function was adequate without extrinsic innervasi. Full extrinsic mainly through kolinergik and adrenergic fibers. This kolinergik fibers causes contraction, and adrenergic fibers causing inhibition.
In patients with Hirschsprung's disease, ganglion cells can not find that the intrinsic control decreases, causing an increase extrinsic neural control. Innervasi of the adrenergic system and kolinergik increased 2-3 times compared to normal innervasi. Adrenergic system expected to dominate kolinergik system, leading to increased intestinal smooth muscle tone. With the loss of intrinsic neural control, increased tone was not balanced and may cause an imbalance of smooth muscle contractility, peristaltic, uncoordinated, and eventually, obstruction fugsional
FREQUENCY
United States
Hirschsprung disease occurs in about 1 per 5400-7200 births.
International
It is not known the correct frequency for the whole world, although some international studies reported incidence of about 1 case from 1500 to 7000 births.
Mortality / Morbidity
* Approximately 20% of infants will have abnormalities involving the neurological system, cardiovascular, urological, or gastrointestinal.
* Hirschsprung's disease has been associated with the disease known below:
o Down Syndrome
o Syndrom Neurocristopathy
o Waardenburg-Shah syndrome
o Deaf-blind yemenite syndrome
o Piebaldisme
o Goldberg-Shprintzen syndrome
o Multiple endocrine neoplasia type II
o Syndrome congenital central hypoventilation
* Megacolon aganglionik not resolved in infancy will lead to increased mortality by 80%. Operative mortality in the intervention procedure is very low. Even padaUntreated aganglionic megacolon in Infancy may result in a mortality rate of as much as 80%.
* Possible complications that can occur which anastomose leakage (5%), stricture anastomose (5-10%), intestinal obstruction (5%), pelvic abscess (5), and wound infections (10%). Long-term complications including obstructive symptoms, inkontinensi, chronic constipation, and enterocolitis, complications are mostly found in patients with long segment aganglionik. Although most patients will have this problem after surgery, the long-term studies have shown bahw more than 90% of children will experience a significant improvement. Patients with long segment proved aganglionik have a worse outcome.
Ras
Hirschsprung disease has no particular race predileksi on.
Gender
Hirschsprung disease is more common in men than women, with a ratio of about 4:1. However, the long segment aganglionik often found in female patients.
Age
* Age in which patients diagnosed as having Hirschsprung's disease decreased since the last century. In early 1900, the median age of 203 years beginning in 1950 until 1970, the median usian be 206 months.
* Currently, about 90% of patients with Hirschsprung's disease can be diagnosed was the perinatal period.
Clinical
Anamnesis
* Approximately 10% of patients have a history of similar illness in the family. This situation is more frequently found in patients with a more aganglion segment length.
* Hirschsprung's disease should be suspected in children who experienced delays in issuing meconium or in children with a history of chronic constipation since birth. Other symptoms include bowel obstruction with bile vomiting, abdominal distension, decreased appetite, and stunted growth.
* Prenatal ultrasound that showed a picture of the obstruction is rare, except in cases involving all parts of the colon.
* Children with older age usually have chronic constipation since birth. They also show evidence of weight gain poorly.
* About 10% of children who came with diarrhea caused by enterocolitis, which is thought to relate to the growth of bacteria due to stasis. This condition can progress to perforation of the colon, causing sepsis.
* In the study involving 259 patients, Menezes et al reported 57% of patients came with symptoms of intestinal obstruction, 30% with constipation, 11% with enterocolitis, and 2% with intestinal perforation.
Physical Examination
* Physical examination of the neonate usually can not make a diagnosis, only shows the existence of abdominal distension and / or spasm of the anus.
* Ani Imperforata low position with perineal hole may have a similar picture with Hirschsprung patients. A thorough physical examination can distinguish between them.
* In older children, abdominal distension caused by the inability to release flatus seldom found
Differential Diagnosis
- Constipation
- Ileus
- Iritable Bowel Syndrome
- Bowel motility disorders
Examination Support
Laboratory examination
* Blood chemistry: In most patients the findings of electrolytes and renal panel usually within normal limits. Children with diarrhea had results consistent with dehydration. This examination can help guide the management of fluid and electrolytes.
* Blood Routine: This examination is performed to determine hematocrit and platelets preoperatif.
* Profile coagulation: The tests were to ensure no blood clotting disorder that needs to be corrected before surgery.
Radiology examinations
* Foto Polos Abdomen may indicate a distended bowel loop with the air in the rectum
* Barium enema
o Do not clean the distal colon by enema before entering the contrast enema as this would blur the image on the transition zone region.
o diletakksan catheters in the anus, without mengembungkan balloon, to avoid ambiguous transition zone and at risk of perforation.
o Photos taken immediately after injection of contrast and taken again 24 hours later.
o Colon narrowing distal to the proximal part of the dilated gambara Hirschsprung disease classification. However, radiological findings in neonates is more difficult to interpret and often fail to show the transition zone.
o other radiological picture that leads to Hirschsprung's disease is the retention of the contrast more than 24 hours after a barium enema performed
Other examinations
* Manometri anorectal
o Manometri detect anorectal reflex relaxation of the lumen internalsphincter after rectal distension. Inhibitorik normal reflex is estimated not found in patients with Hirschsprung's disease.
first Swenson kai o use of this examination. In 1960, repairs will be done but is less desirable because it has many limitations. Normal physiological status and sedation is often needed is important. False positive results have been reported to reach 62% of cases, and false negatives were reported as much as 24% of cases.
o Because of these limitations and the questionable reliability, anorectal manometri rarely used in the United States
* Because cardiac malformations (2-5%) and trisomy 21 (5-15%) was also associated with congenital aganglionosis, and genetic tests recommended kardiologis
Procedure
* Rectal biopsy
o Hirschsprung definitive diagnosis is by biopsy of rectal, namely the discovery ketidakberaadan ganglion cells.
o definitive method to retrieve the network to be examined is by biopsy of full-thickness rectal
o Specimens should be taken a minimum distance of 1.5 cm above the dentata line because aganglionosis is usually found at the level
o Lack of this investigation is the possibility of hemorrhage and the formation of scar tissue and the use of public anastesia during the procedure in done.
* Simple rectal suction biopsy
o More recently, simple rectal suction biopsy technique has been used as a network to retrieve histological examination
o rectal mucosa and submucosal sucked through the engine cylinders and a special knife to cut the desired tissue.
o Benefits of this inspection is easily performed on the patient's bed.
o However, the diagnosis of Hirschsprung's disease pathological samples taken from the simple rectal suction biopsy is more difficult than in tissue taken with the technique of full-thickness biopsy
o Ease of diagnosing has been updated with the use of coloring asetilkolinesterase, which quickly coloring hypertrophy of nerve fibers along the lamina propria and muscularis propria in the network.
Histological discovery
Both myenteric plexus (Auerbach) and submucosal plexus (Meissner) not found in the muscular layer of the intestinal wall. Nerve fibers undergoing hypertrophy seen with staining asetilkolinesterase also found along the lamina propria and muscularis propria. Now there has been a calretinin immunohistochemistry examination which had been used for histological examination aganglionik intestine, and there are studies that have concluded that the investigation was likely to be more accurate in detecting asetilkolinesterase than aganglionosis.
Management
Medical treatment
The general objectives of this treatment include the 3 main things: (1) to handle the complications of Hirschsprung disease is not detected, (2) as a temporary management before definitive reconstructive surgery performed, and (3) to improve bowel function after reconstruction surgery.
* Management of complications is directed at fluid and electrolyte balance, avoiding excessive distension, and overcoming systemic complications, such as sepsis. Therefore, hydrasi intravenous, dekompressi nasogastrik, and if indicated, intravenous antibiotics have a major role in the initial medical management.
* Cleaning the colon, namely by doing rectal irrigation with large hollow tubes and fluid for irrigation.
* The liquid to prevent electrolyte imbalance.
* Irrigation routine colon and prophylaksis antibiotic therapy has become the procedure to reduce the risk of enterocolitis.
* Injection of BOTOX in the internal sphincter proved to trigger a pattern of normal bowel movements in post-operative patients.
Handling operative
Hirschsprung operative handling begins with early diagnosis, which usually requires biopsy of full-thickness rectal. In general, initial management is to create a colostomy and when the child grows and has a weight more than 10kg, definitive surgery can be done.
Management standard was developed in 1950 after reporting high rates of leakage and stricture in a single procedure described by Swenson. However, with progress anastesia a safer and more hemodinamika monitoring progress, withdrawal procedures, without a colostomy more frequently used. Contraindications for this single procedure is maximal dilatation of the proximal colon, entercolitis weight, perforation, malnutrition, and inability to determine accurately the transitional zone.
For the first time neonates treated with colostomy, originally identified and the transition zone colostomy performed on the proximal part of this area. The presence of ganglion cells in the colostomy location must be confirmed by frozen-section biopsy. Both loop or end-stoma can be done, usually depending on the surgeon's preference.
Some definitive procedure has been used, all of which have given excellent results when performed by experienced surgeons. 3 types of techniques are frequently used procedure Swenson, Duhamel, and Soave. Whatever the technique performed, colonic cleansing before definitive surgery is very important.
* Procedure Swenson
o Procedure Swenson is the first definitive technique used to treat Hirschsprung's disease
o Segments until aganglionik resectable sigmoid colon and oblique anastomosis performed between the normal colon with the distal rectum
* Procedure Duhamel
o Duhamel procedure was first introduced in 1956 as a modification of Swenson procedure
o The main point is the approach used retrorektal and some parts of the aganglionik retained rectum.
o Colon aganglionik resectable up to the rectum and rectum sewn. Proximal part of the intestine and then positioned on retrorektal space (between the rectum and the sacrum), and end-to-side anastomosis performed on the remaining rectum
* Soave Procedure
O Soave procedure was introduced in 1960, the point is to remove the mucosal and submucosal of the rectum and colon ganglionik pulled toward the end of the rectum aganglionik muscular.
o Initially, this operation does not include a formal anastomosis, depending on the formation of scar tissue between the segments drawn and intestinal aganglionik. This procedure was later modified by Boley with a primary anastomosis of the rectum.
* Myomectomy anorectal
o For children with Hirschsprung's disease with a very short segment, remove some parts of the midline posterior rectal other operating alternatives
o This procedure is 1 cm away ekstramukosal rectal wall that started around the proximal dentate line.
o The mucosa and submucosal maintained and closed.
* Laparaskopik as a management approach Hirschsprung's disease was first described in 1999 by Georgeson. Transition zone is determined laparaskopik determined initially, followed by mobilization of the rectum below the peritoneal. Transanal mucosal dissection performed, followed by removing the rectum through the anus and the anastomosis. Functional results seemed similar to the open technique based on short-term results
Diet
* Foods high in fiber and contains fresh fruit can optimize the function of post-operative bowel in some patients.
Activities
Limit physical activity for about 6 weeks for wound healing is well
Medication
The purpose of farmakoterapi to mengeradiksi infection, reduce morbidity, and reduce complications.
Antibiotics
Antimicrobial therapy must be comprehensive and cover all pathogens associated with clinical conditions. Selection of antibiotics should also be guided by blood culture tests and sensitivity.